√100以上 yield formula chemistry 309013-Theoretical yield formula organic chemistry
Yield}$$\times$ 100% Percentage yield = $\frac{06}{14}$$\times$ 100% Percentage yield = 429% The percentage yield of this reaction is 429%, Scientist tries to choose reactions with a high percentage yield or high atom economyUse the mass of product obtained to determine the percent yield percent yield = grams of product obtained X 100% theoretical yield (in grams) or convert the mass of product obtained to the moles of products obtained (using the MW of the product) to determine the percent yieldThe formula for calculating the percent yield is Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100% Let's assume that you obtained an actual yield of 850 grams Then, the percent yield would be Percentage yield of NaCl = 850 grams ÷ 993 grams × 100% Percentage yield of NaCl = 8559%

How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
Theoretical yield formula organic chemistry
Theoretical yield formula organic chemistry-The question reads that the actual yield from 100 grams of Calcium Hydroxide is 50 grams of Calcium Oxide Hence, the percent yield is given by Percent Yield = Actual Yield/Theoretical Yield xSo, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)



Conversion Chemistry Wikipedia
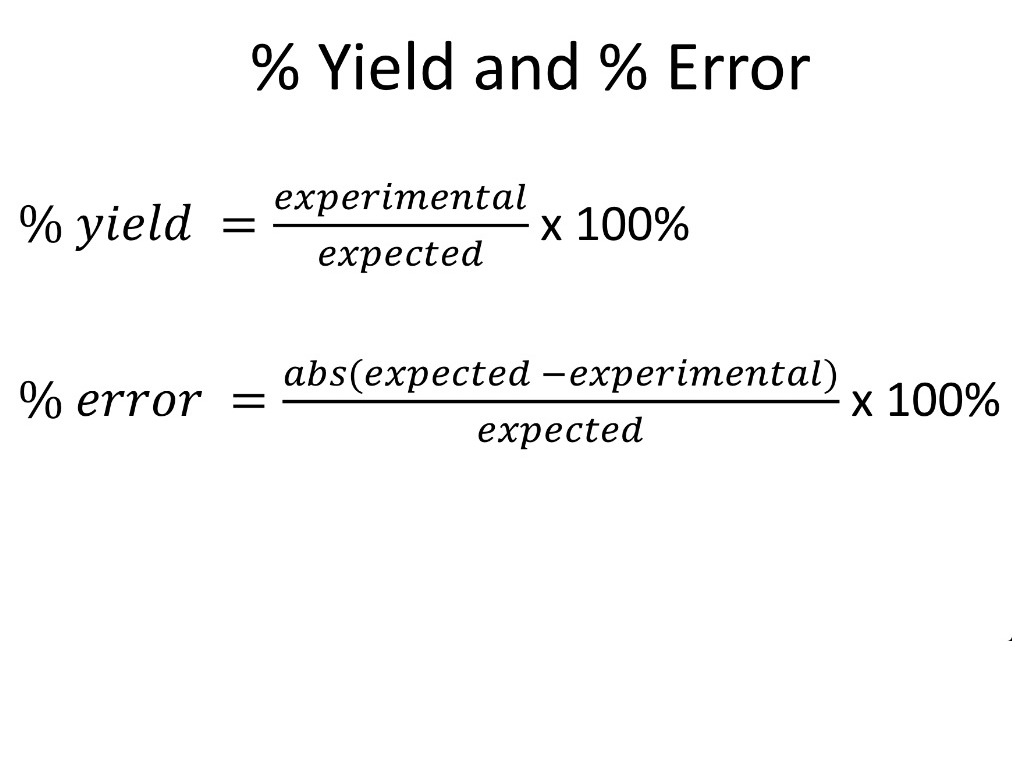
Percent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%The absolute yield can be given as the weight in grams or in molar (molar yield) while the fractional yield or relative yield or percentage yield is calculated by dividing the amount of the obtained product in moles by the theoretical yield in moles The percentage yield is obtained by multiplying the fractional yield by 100%So, all you need to put the values into the percent yield formula The percent yield equation is percent yield = (actual yield/theoretical yield) x 100% percent yield = (15 g MgO/19 g MgO) x 100% = 79 % So, the percent yield of magnesium oxide = 79 % Frequently Ask Questions What is the actual yield of a reaction?
Substitute the values in the corresponding formula Percentage yield = $\frac{Actual\;6O 2 C 6 H 12 O 6 6 → CO 2 6H 2 O After balancing the equation, now we know that after the actual reaction if there is no loss of reactants, our product will be six molecules of carbon dioxide and six molecules of water It is the theoretical yield However, it is impossible for a ration to give 100 percent yieldUsing this theoretical yield and the provided value for actual yield, the percent yield is calculated to be percent yield = ( actual yield theoretical yield)×100 percent yield = ( actual yield theoretical yield) × 100 percent yield = ( 0392 g Cu g Cu) ×100 percent yield = ( 0392 g Cu g Cu) × 100
Eg, volume, if the product is a gas)What is the theoretical yield in grams for this reaction?Yield Burning The illegal practice of underwriters marking up the prices on bonds for the purpose of reducing the yield on the bond This practice, referred to as "burning the yield," is done



Limiting Reagent Percent Yield Stoichiometry General Chemistry Lecture 1140 Dr Sundin Uwp



Calculation Methods Of Yield Strength And Ultimate Tensile Strength By Download Scientific Diagram
Percent yield or percentage yield is the ratio of the actual yield and the theoretical yield of a chemical reaction The experimental yield is divided by the theoretical yield and multiplied by 100 to be calculated as the percent yield If the theoretical yield and the experimental yield are same then the percent yield will be 100%The following chemical reaction takes place ∑ i = 1 n ν i A i = ∑ j = 1 m μ j B j {\displaystyle \sum _{i=1}^{n}\nu _{i}A_{i}=\sum _{j=1}^{m}\mu _{j}B_{j}} , where ν i {\displaystyle \nu _{i}} and μ j {\displaystyle \mu _{j}} are the stoichiometric coefficientsUsing the theoretical yield equation above, we know our grams of hydrogen will yield x 1/2 x 1/5 x 250 = 500 grams of product How to Calculate Theoretical Yield in Chemistry The individual steps of the process of calculating theoretical yield looks like this



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Molecular Formulas And Nomenclature
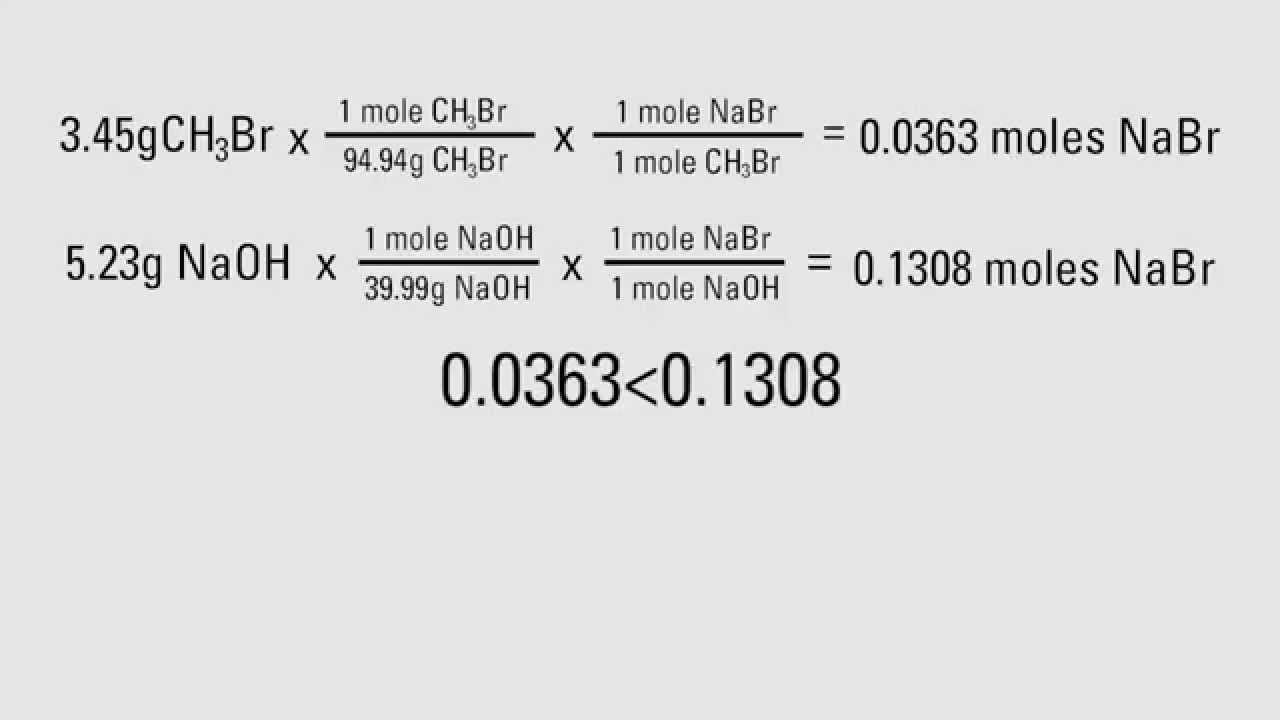
If 4AI 3O2 = 2 AI2O3 , limiting reactant is AI Divide number of AI moles by two, since it takes four to make 2 AI2O3) Finally, to get a theoretical yield, you'll need to multiply the number of moles of the product by the molecular weight of the productTo calculate the theoretical yield, consider the reaction (2) CO ( g) 2 H 2 ( g) → CH 3 OH ( l) (3) 280 40 3 ( stoichiometric masses in g, kg, or tons) 12 t o n s H 2 × 3 C H 3 O H 40 H 2 = 96 t o n s C H 3 O H Thus, the theoretical yield from 12 metric tons (12x10 6 g) of hydrogen gas is 96 tonsDefinition of Conversion, Selectivity and Yield This is the definition that we used in our simulation work



A Simple Guide On How To Calculate Theoretical Yield Detox Fast Wine Folly Wine Map



How To Calculate The Yield For An Incomplete Reaction Chemistry Stack Exchange
Calculating percentage yield The percentage yield is calculated using this formula \ Percentage~yield = \frac {yield~obtained} {theoretical~yield} \times 100\ For example, if the predictedIt's given that we have 021 moles, divided by what was expected which was 03 moles and multiplied by 100% This is 70% and that is the yield of this reaction So, in order to find the yield, can divide the masses to do the actual yield in mass divided by the theoretical yield you can have the moles divided by molesTheoretical Yield Formula Solved Examples & Practice Questions In theory, we can always predict the amount of desired product that will be formed at the end of a chemical reaction Assuming that the reaction will go to completion we can predict this amount of product from the stoichiometric coefficients of the balanced chemical equation



How To Calculate Percent Yield Math Wonderhowto



Unit 8 Percent Yield Calculations Ppt Download
You calculated the AVERAGE yield, not the overall yield Marc Vidal is right, you should the yield of every step as fractions of 1, multiply them (but only over a linear sequence) and multiply byAbout Add CO 2 to a Reaction Add Reagents Clean Glassware Degas Solvents Get a Good 1 H NMR Spectrum Grow XRay Quality Crystals Handle Azides Improve Yield Manage an Emulsion Monitor by TLC Prepare Jones Reagent Prepare LDA Present a Talk Purify by Crystallization Purify by Distillation Remove Residual Water Run a Flash Column Run a Prep TLCIn reality, most reactions are not perfectly efficient If you perform the experiment, you'll end up with a smaller amount, the actual yield To express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured)


How To Calculate Theoretical Yields Youtube



Pin By Jesus Gomez On Estequiometria Chemistry Class Science Chemistry Worksheets
Before performing chemical reactions, it is helpful to know how much product will be produced with given quantities of reactants This is known as the theoretical yieldThis is a strategy to use when calculating the theoretical yield of a chemical reactionBy using the lesson called How to Calculate Percent Yield Definition, Formula & Examples, you can expand what you know about this concept in chemistry and review Understanding chemical reactionsThe theoretical molar yield is mol (the molar amount of the limiting compound, acetic acid) The molar yield of the product is calculated from its weight (132 g ÷ g/mol = 15 mol) The % yield is calculated from the actual molar yield and the theoretical molar yield (15 mol ÷ mol × 100% = 75%) citation needed



Percent Yield Percent Purity Solutions Examples Videos



Reaction Yield Protocol
In chemistry, we have theoretical yield, which is the amount of the product calculated from the limiting reactant The limiting reactant is the reactant in the chemical reaction which limits the\\ Percent\;Yield = \frac{Actual\;Yield}{Theoretical\;Yield} \times {100} \ As soon as you will rearrange the formula then the theoretical yield can be calculated quickly Before you start with some chemical reaction, this is necessary to check how product can be produced by given quantities of reactantsLatex\text{percent yield}=\frac{\text{actual yield}}{\text{theoretical yield}}\times 100\%/latex Actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property;



Solved The Chemistry Of Recycling Percent Yield The Fina Chegg Com


Percent Yield And Percent Error Science Chemistry Percent Yield Percent Error Showme
Grams O 2 = 80 grams O 2 To produce 90 grams of water, 10 grams of hydrogen gas and 80 grams of oxygen gas are needed Theoretical yield calculations are straightforward as long as you have balanced equations to find the mole ratios needed to bridge the reactants and the productTheoretical Yield Formula Questions 1 Determine the theoretical yield of H 2 O (in moles) in the following reaction, if 25 moles of hydrogen peroxide are decomposed 2H 2 O 2 → 2H 2 O O 2 Answer In this reaction there is only one reactant (H 2 O 2) so it must be the limiting reactant Stoichiometry will be used to determine the moles of water that can be formedPercent yield = purified percent yield = amount of P (g) theoretical yield (g) •100 Percent yield (if stoichiometry is 11) = amount of P (mol) amount of LR (mol) •100 Complicated balanced equations are uncommon in organic chemistry Many organic reactions have a stoichiometry of 11 in the balanced equation


Theoretical Yield Calculator
:max_bytes(150000):strip_icc()/148302528-56a12f323df78cf77268383a.jpg)


Percent Yield Definition And Formula
The equation for percent yield is percent yield = actual yield/theoretical yield x 100% Let me show you how this works with an actual chemical reaction Example OneChemistry Chemical Word Equations Directions Write a balanced chemical equation for each of the word equations below 1 aqueous sodium chloride reacts with aqueous lead (II) nitrate to yield a lead (II) chloride precipitate and aqueous sodium nitrateScience AP®︎/College Chemistry beta Chemical reactions Stoichiometry Stoichiometry Stoichiometry Worked example Calculating amounts of reactants and products Limiting reactant and reaction yields This is the currently selected item



Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab


The Stoichiometry Of Product Formation And Percent Yield
The percent yield is simply the actual yield divided by theoretical yield multiplied by 100 Actual yield is the amount of product you actually got while theoretical is the maximum possible yield Be sure that actual and theoretical yields are both in the same units so that units cancel in the calculationTheoretical yield (in grams) or convert the mass of product obtained to the moles of products obtained (using the MW of the product) to determine the percent yield percent yield = moles of product obtained X 100% theoretical yield (in moles)Conversion and its related terms yield and selectivity are important terms in chemical reaction engineering They are described as ratios of how much of a reactant has reacted (X — conversion, normally between zero and one), how much of a desired product was formed (Y — yield, normally also between zero and one) and how much desired product was formed in ratio to the undesired product(s


Calculating Percentage Yield
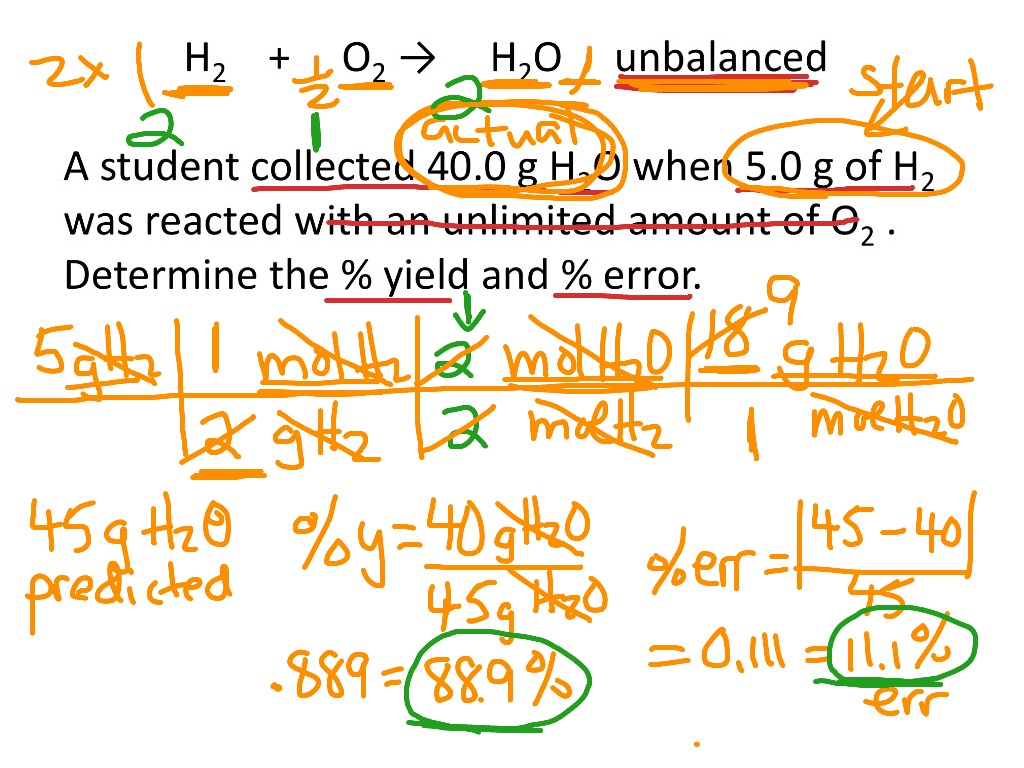


Percent Yield And Percent Error Calculations Science Chemistry Percent Yield Percent Error Showme
The amount of product predicted by stoichiometry is called the theoretical yield, whereas the amount obtained actually is called the actual yield A reaction yield is reported as the percentage of the theoretical amount The formula for percentage yield is given by Percentage yield= (Actual yield/theoretical yield )x100The actual yield is expressed as a percentage of the theoretical yield This is called the percent yield To find the actual yield, simply multiply the percentage and theoretical yield together Answer linkThe percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage (1291) Percent Yield = Actual Yield Theoretical Yield × 100 % Percent yield is very important in the manufacture of products Much time and money is spent improving the percent yield for chemical production


Limiting Reagents
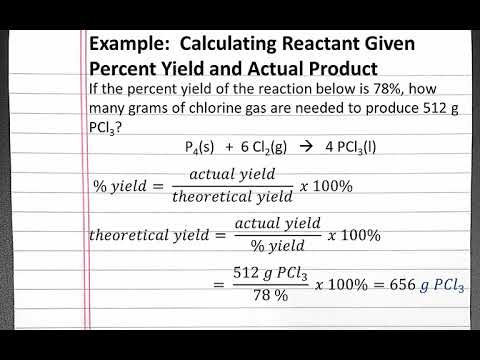


Chemistry 101 Calculating Reactant Given Percent Yield And Actual Product Youtube
1 mole of salicylic acid gives 1 mole of aspirin So, 0725 moles gives 0725 moles of aspirin 0725 moles of aspirin = 0725 × 180 g = 1305 g So, the calculated mass of the reaction is 1305 g Step 4 Calculate the percent yield The actual mass obtained is 1212 g So, the percent yield = 1212 ÷ 1305 × 100% = 929% How to calculate the percent yield of a chemical reaction?Onestep reaction yield This is expressed as the relative yield (in percentage, %) and results from dividing the moles of the product between the theoretical moles of the product (maximum amount that would result from the product if the entire amount of limiting reagent is consumed in the reaction) (27) A → B Yield ( %) = mol of the product yielded mol of the product expected × 100The formula to determine actual yield is simple you multiply the percentage and theoretical yield together The limiting reagent and excess reagent In a chemical reaction, you have both limiting reagents and excess reagents
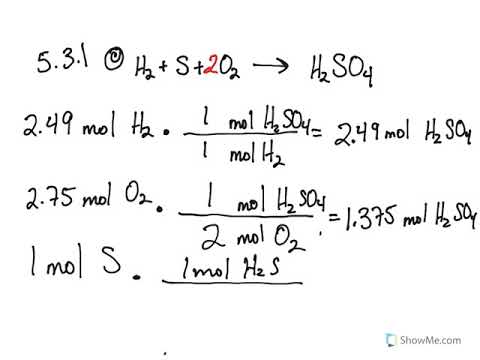


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



How To Calculate Percent Yield In Chemistry 15 Steps
Yield Calculations Chemistry Tutorial Key Concepts Yield is the mass of product formed in a chemical reaction Actual yield is the mass of product formed in an experiment or industrial process Theoretical yield is the mass of product predicted by the balanced chemical equation for the reactionPercent Yield Formula The equation for percent yield is percent yield = (actual yield/theoretical yield) x 100% Where actual yield is the amount of product obtained from a chemical reaction theoretical yield is the amount of product obtained from the stoichiometric or balanced equation, using the limiting reactant to determine productChemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actual


8 6 Limiting Reactant Theoretical Yield And Percent Yield From Initial Masses Of Reactants Chemistry Libretexts



Percentage Yield Lab Answers Schoolworkhelper
Latex\text{percent yield}=\frac{\text{actual yield}}{\text{theoretical yield}}\times 100\%/latex Actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property;Eg, volume, if the product is a gas)Guidelines for Yield Reporting in Lab Reports Always remember to present your yields and percent yields in the Results Tables Base the percent yield on the theoretical yield (Not on the "expected yield" Please note that the theoretical yield is never the "expected yield" as we never expect 100% yield in a chemical reaction)
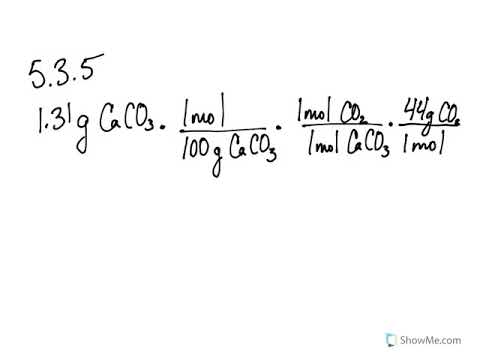


5 3 Calculating Reaction Yields Problems Chemistry Libretexts
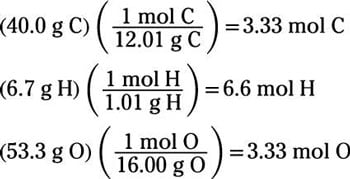


How To Calculate The Empirical Formula Of A Compound Dummies
The theoretical yield refers to the amount that should be form when the limiting reagent is completely consumed The actual yield is expressed as a percentage of the theoretical yield This is called the percent yield To find the actual yield, simply multiply the percentage and theoretical yield together Answer link



Process Chemistry Wikipedia



Solved Calculate Theoretical Yield For Each Of The Possib Chegg Com
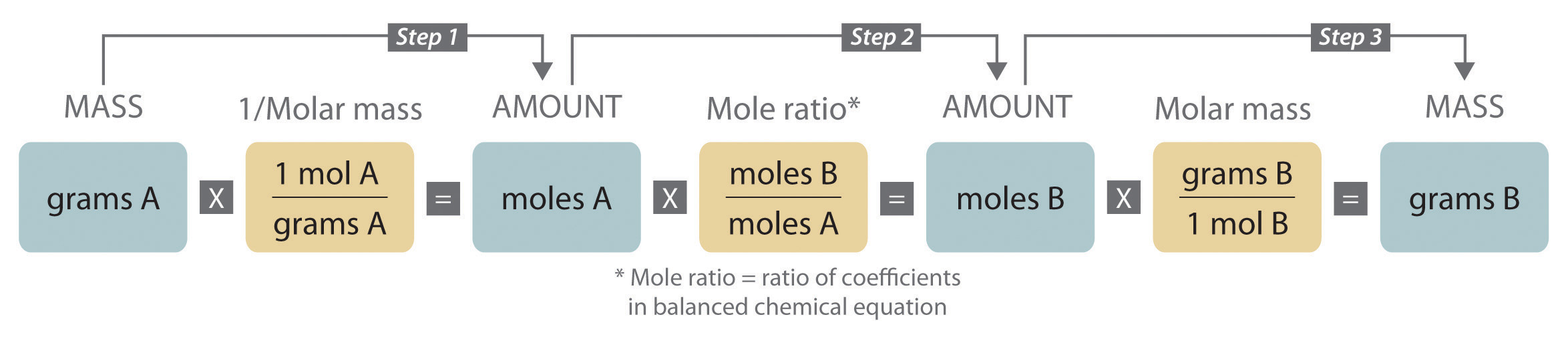


Mass Relationships In Chemical Equations



Calculating Amounts Of Reactants And Products Worked Example Video Khan Academy



C2 Chemistry Powerpoint Presentation Free Online Download Ppt Aertax
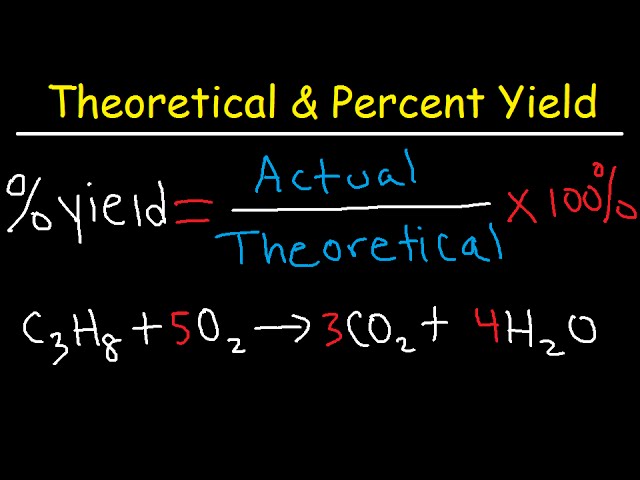


How To Calculate Theoretical Yield And Percent Yield Youtube


Calculation Of Theoretical Yield Organic Chemistry I 212 01



How To Calculate Percent Yield 3 Ways To Solve Chemistry Problems Tripboba Com



Yields Introductory Chemistry



Reaction Percent Yield Introduction And Practice Exercises



Yield Calculations Faculty Staff Sites



Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
/GettyImages-82845115-1--56a134e33df78cf772686225.jpg)


Stoichiometry Definition In Chemistry



Yield Calculations Faculty Staff Sites


Calculation Of Theoretical Yield Organic Chemistry I 212 01
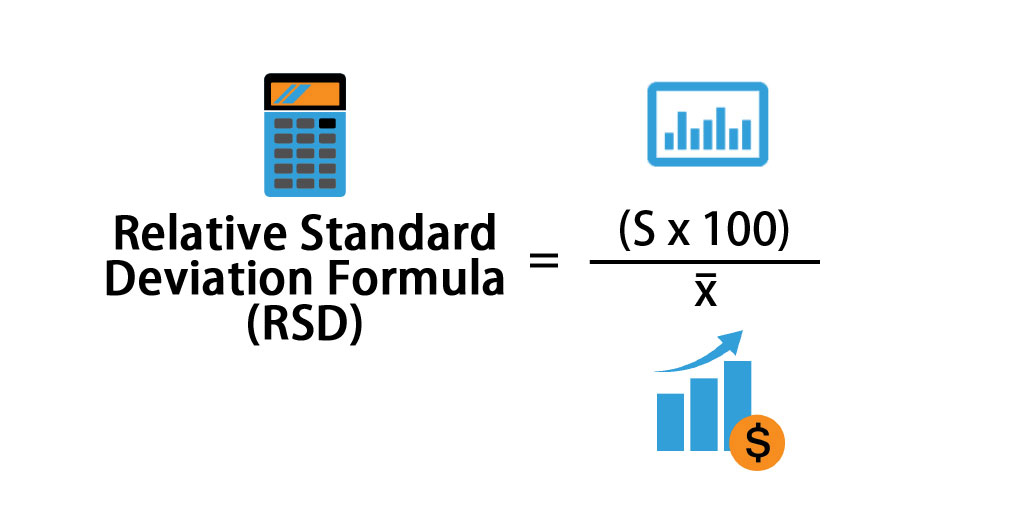


Relative Standard Deviation Formula Rsd Calculator Excel Template


Secure Media Collegeboard Org Digitalservices Pdf Ap Apcentral Ap15 Chemistry Q2 Pdf


3



Percent Yield Chemistry Video Clutch Prep



Howto How To Find Percent Yield Without Actual Yield



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com
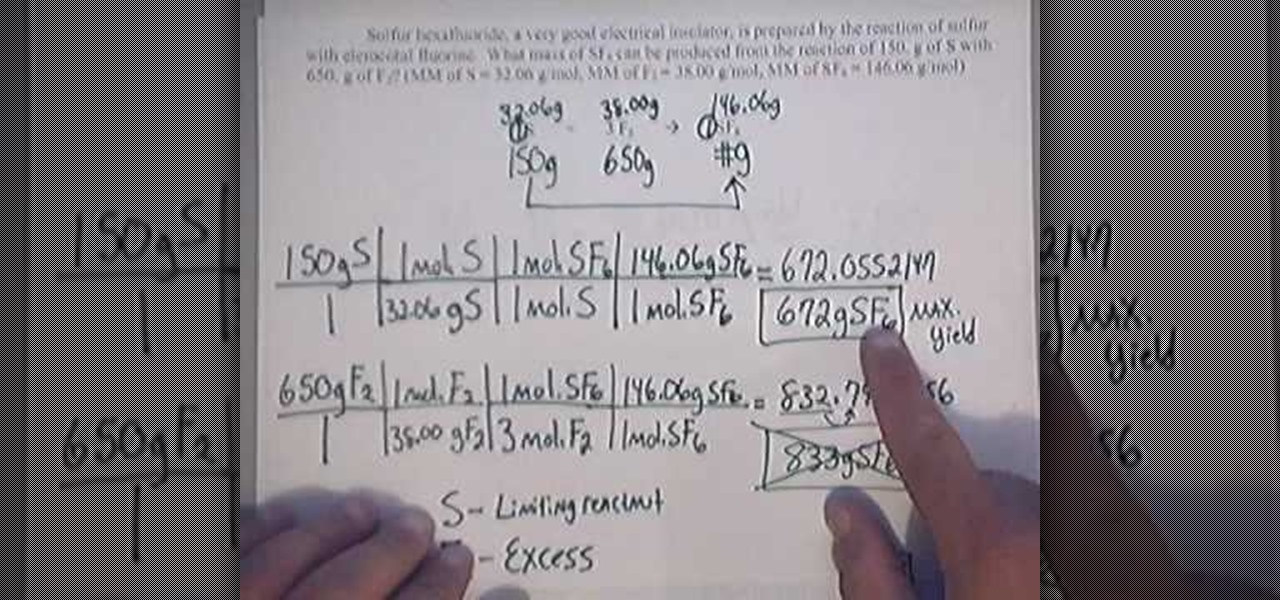


How To Calculate Percent Yield Math Wonderhowto
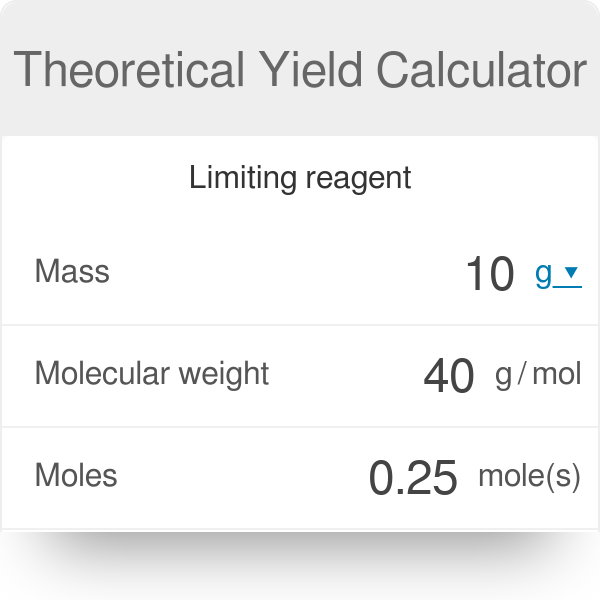


Theoretical Yield Calculator


Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau


Q Tbn And9gcsbwel4mzamey6ite9bp 08j Tpx0zptf7ruyvck4xodffyxfeu Usqp Cau



Percent Yield Tutorial Explained Practice Problems Crash Chemistry Academy Youtube



Conversion Chemistry Wikipedia
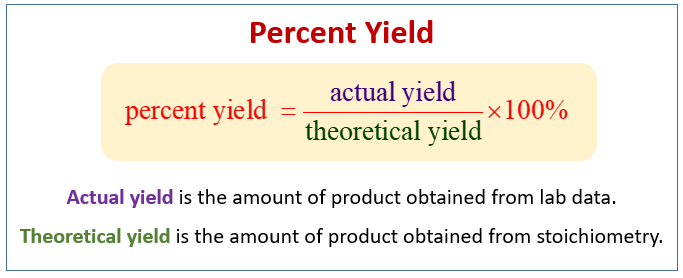


Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
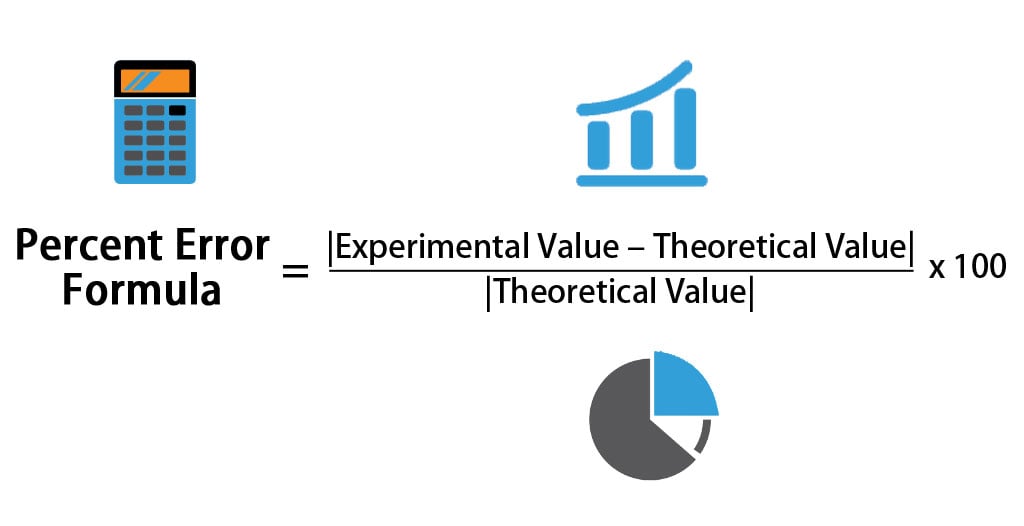


Percent Error Formula Calculator Excel Template


Chemistry Atom Economy And Percentage Yield
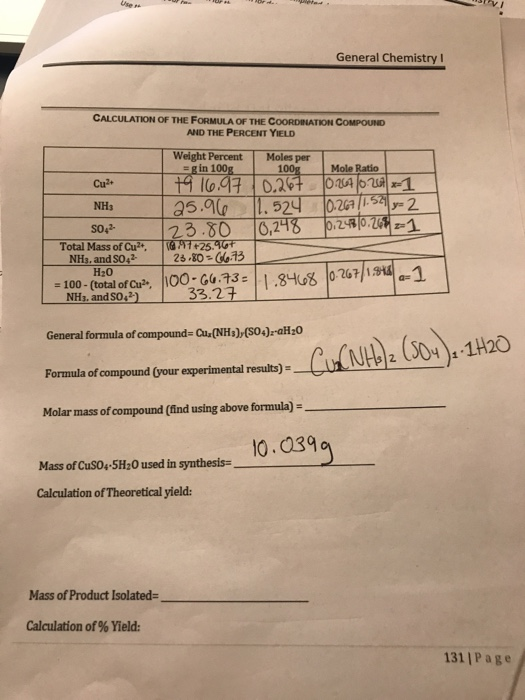


General Chemistry I Calculation Of The Formula Of Chegg Com



Chemistry 6 7 Percent Yield Youtube


Chemistry Atom Economy And Percentage Yield



Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube



Reaction Percent Yield Introduction And Practice Exercises



How To Calculate Theoretical Yield 12 Steps With Pictures
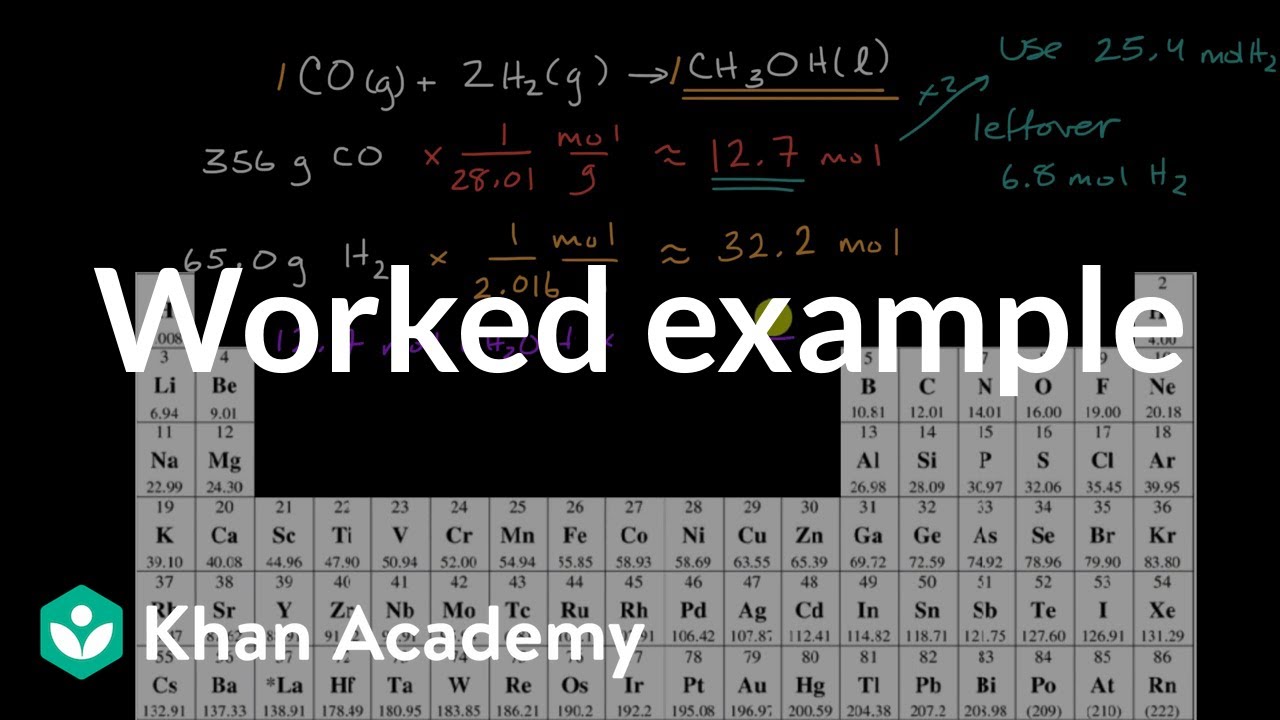


Calculating The Amount Of Product Formed From A Limiting Reactant Worked Example Video Khan Academy
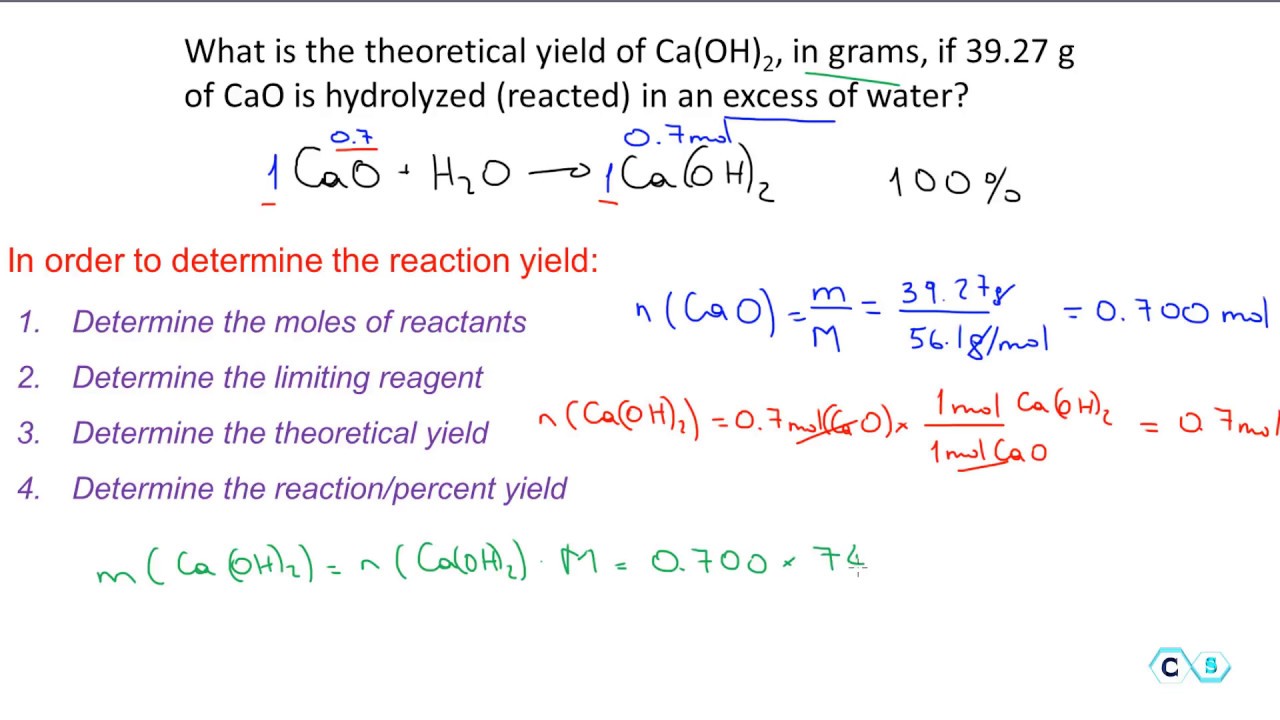


Reaction Percent Yield Introduction And Practice Exercises
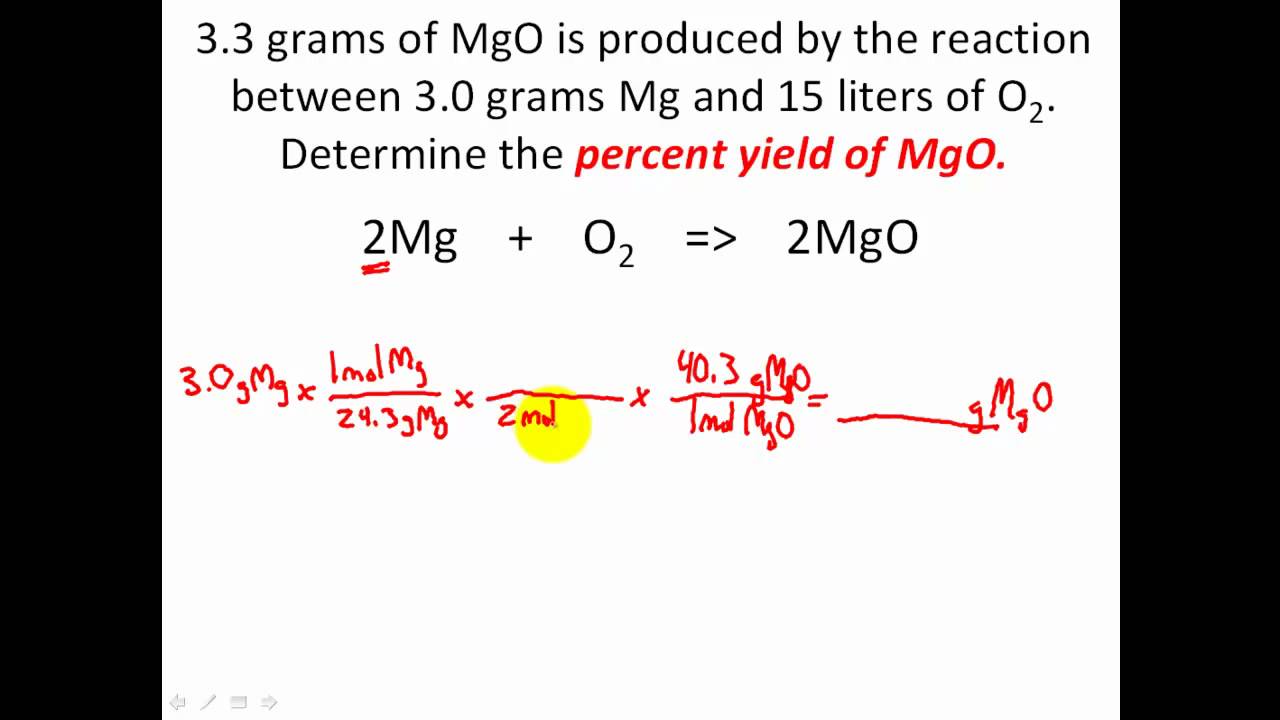


Stoichiometry Solving Percent Yield Stoichiometry Problems Youtube



Level H Chemistry Worksheets Files Moles And Stoichiometry Review



Yields Introductory Chemistry



Calculating Percent Yield Hey Chemistry
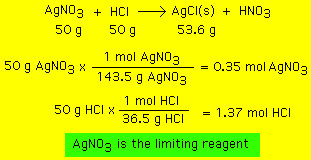


Quantitative Chemistry Theoretical And Percent Yield



Quantitative Chemistry Secondary Science 4 All
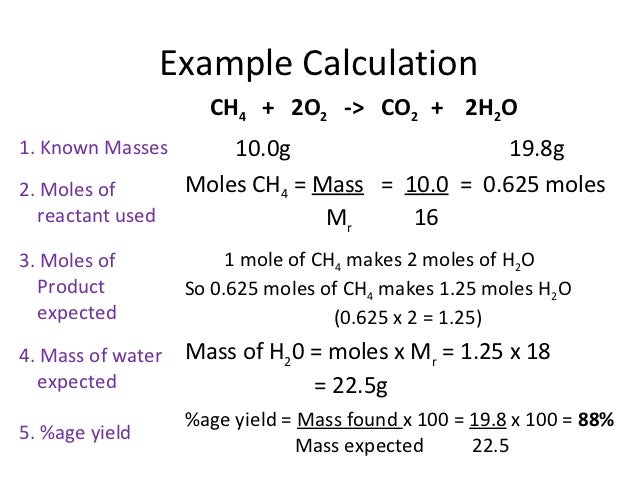


How To Calculate Percentage Yield In Chemistry How To Wiki



Calculating Percent Yield Hey Chemistry
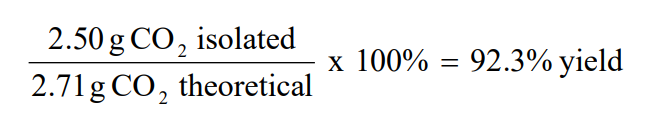


Molecular Formulas And Nomenclature



Moles Percentage Purity And Yield John Vagabond S Physics And Chemistry Blog



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
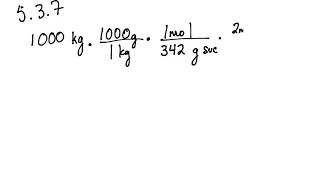


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



How To Find Actual Yield Theoretical Yield And Percent Yield Examples Practice Problems Youtube
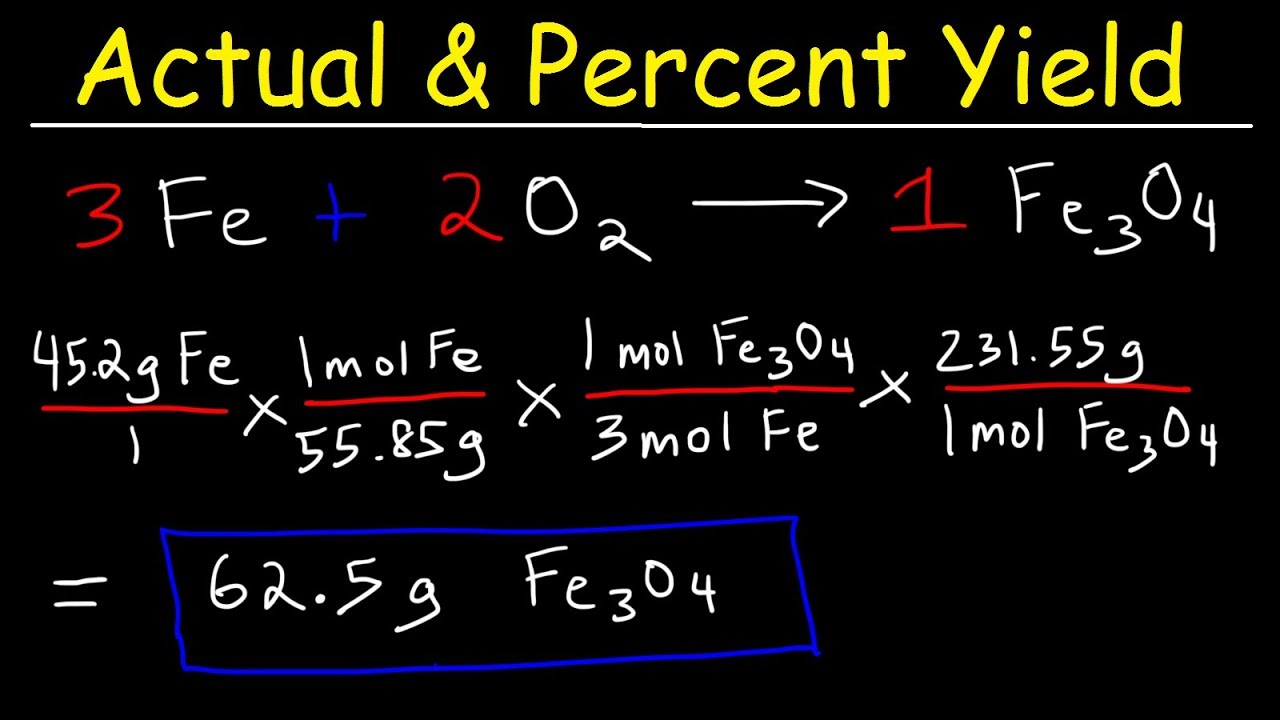


How To Calculate The Percent Yield And Theoretical Yield Youtube



Image Result For Theoretical Yield School Work Chemistry Basic Concepts



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Ib Chemistry Limiting Excess Theoretical And Percentage Yield



Select A Section Introduction Stoichiometry Of Chemical Compounds 3 1 Molecular Masses And Formula Masses 3 2 The Mole And Avogadro S Number 3 3 The Mole And Molar Mass 3 4 Mass Percent Composition From Chemical Formulas 3 5 Chemical



Limiting Reagent And Percent Yield Ppt Download
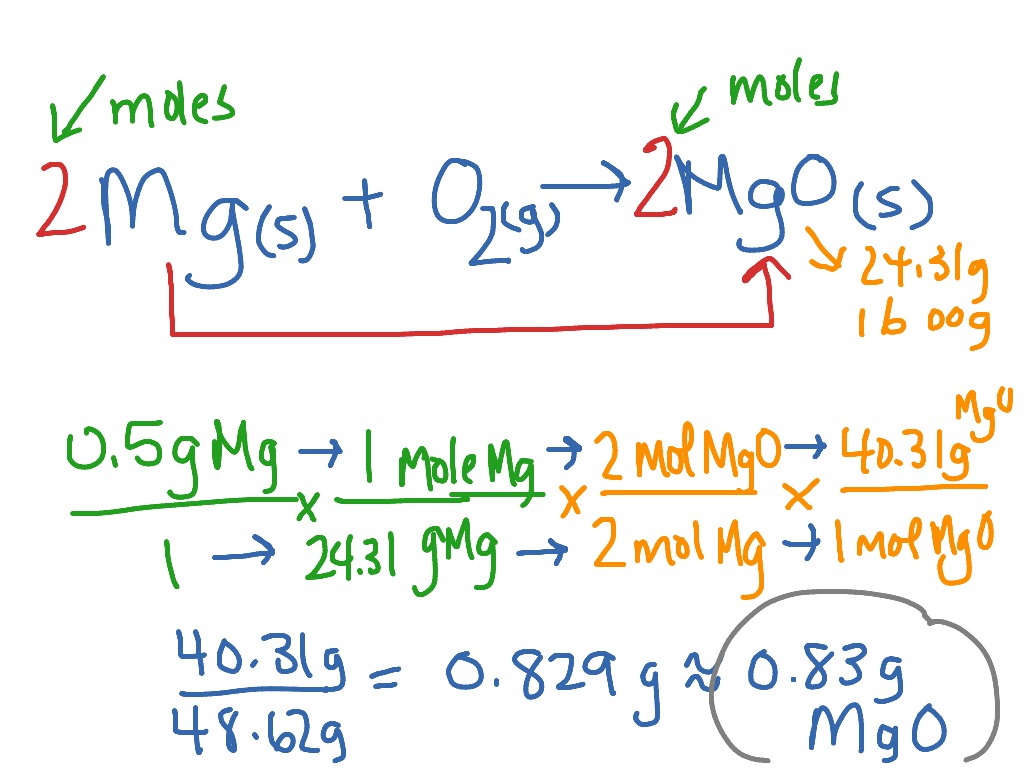


Calculating Theoretical Yield Mgo Showme


Q Tbn And9gcrjyqnm Hsuw1zecxl5b8r5elmfwvpiet 7gqpwpfgze5a6h7ox Usqp Cau



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World
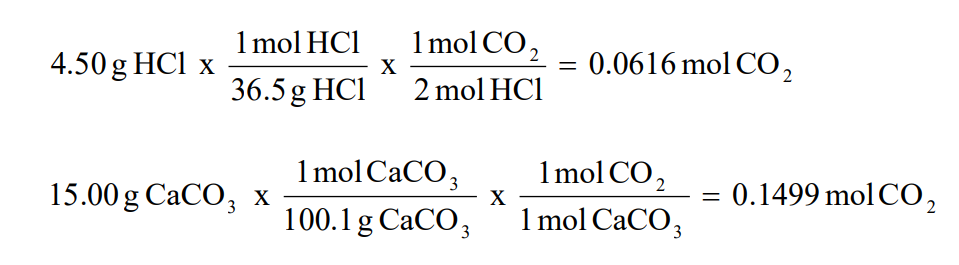


Molecular Formulas And Nomenclature



Grade 10 Chemistry Percentage Purity The Formula Is Actual Mass Percentage Yield 100 Theoretical Yield But Where Do I Get Those Values From This Question Homeworkhelp



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Calculating Percent Recovery Percent Yield



Calculations What You Need To Know Relative Formula Mass Ppt Video Online Download



A Level Chemistry Ocr Salters Yield Wikibooks Open Books For An Open World
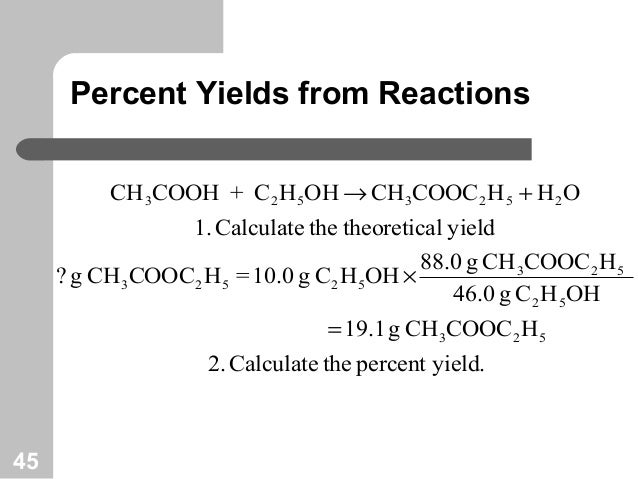


Chemical Equations And Reaction Stoichiometry



Percentage Yield Lab Answers Schoolworkhelper



Multiple Reactions Yield Selectivity Youtube



Question Video Calculating The Percentage Yield Of The Recreation Of Aqueous Copper Sulfate With Zinc Metal Nagwa



Calculating Actual Yield Given The Percent Yield Youtube



Limiting Reagent Stoichiometry Practice Khan Academy
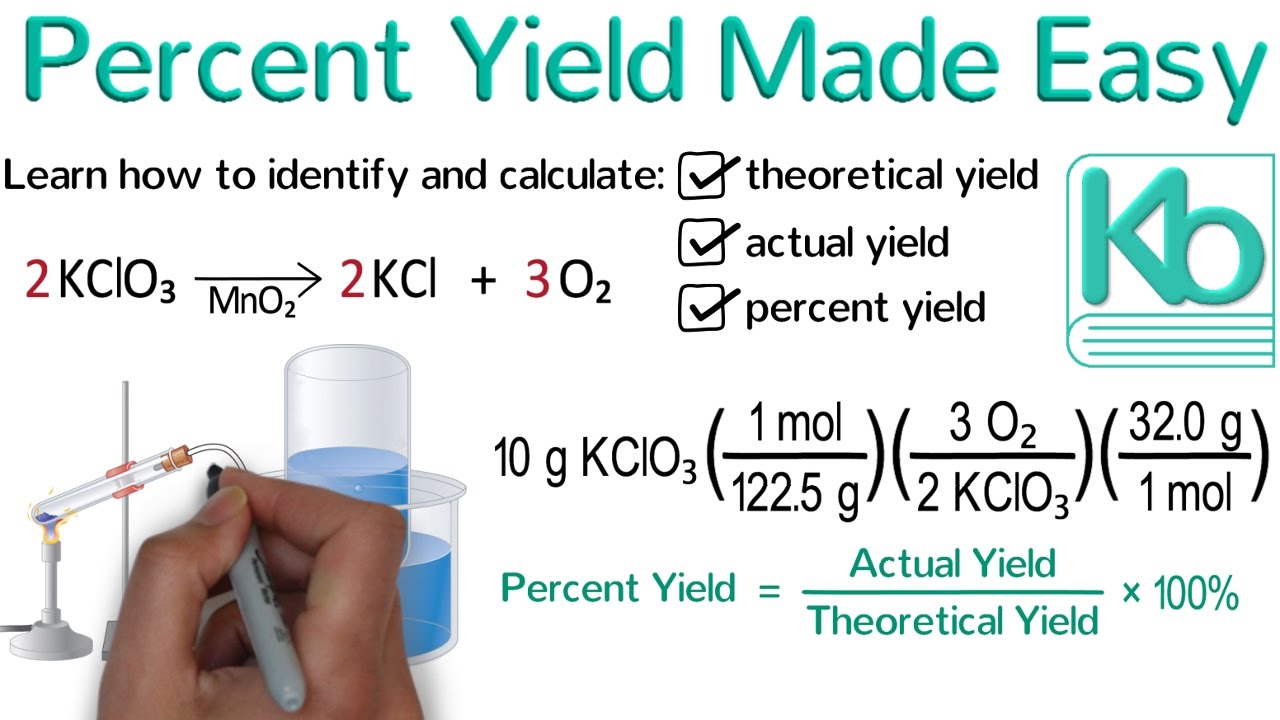


Percent Yield Made Easy Stoichiometry Tutorial Part 4 Youtube



Magnesium Oxide Percent Yield Lab Report Schoolworkhelper


コメント
コメントを投稿